Alternative fuels in the World
Ammonia Cars, ¿how do they work?
A little history of ammonia vehicles

One of the first uses of liquid NH3 as a bus fuel was in Belgium in 1943. Emeric Kroch developed these hybrid ammonia/coal gas engines to keep public transportation running despite extreme diesel shortages during the Second World War.

The X-15 rocket plane broke speed and altitude records in the 1960s, powered by NH3.

In the summer of 2007, this NH3 vehicle toured the United States, from Detroit to San Francisco, powered by a mixture of ammonia and gasoline.
What is ammonia?
Ammonia, at room temperature, is a colorless gas with a very pungent and nauseating odor. It occurs naturally by decomposition of organic matter and is also manufactured industrially. It is easily soluble and evaporates quickly. Ammonia is made up of Nitrogen and Hydrogen, with the chemical formula NH3
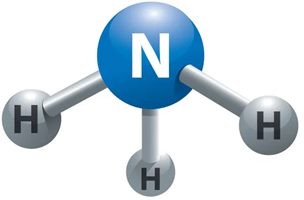
In fact, ammonia is already toxic at low concentrations, when burned it produces NOx (but the technology already exists to reduce these emissions) and needs a "pilot fuel" as it is not easily ignitable.
More than 180 million tons of ammonia are produced each year. For more than 100 years, since the introduction of the Haber-Bosch process, ammonia has been used as a fertilizer for the food industry, as a raw material and coolant for various industrial processes, as a fuel in the aerospace sector, and as a reducing agent in manufacturing processes. reduction of nitrogen oxides. This means that consolidated infrastructures are available for the transport and storage of this substance.
Ammonia is an acrid, colorless gas. It can be easily liquefied due to strong hydrogen bonds and therefore easily transported in thermally insulated containers. Furthermore, ammonia liquefies at very low pressures (0.8 MPa) which is lower than that of hydrogen (up to 70 MPa). It can also be liquefied at -33ºC.
How does an engine with ammonia work?
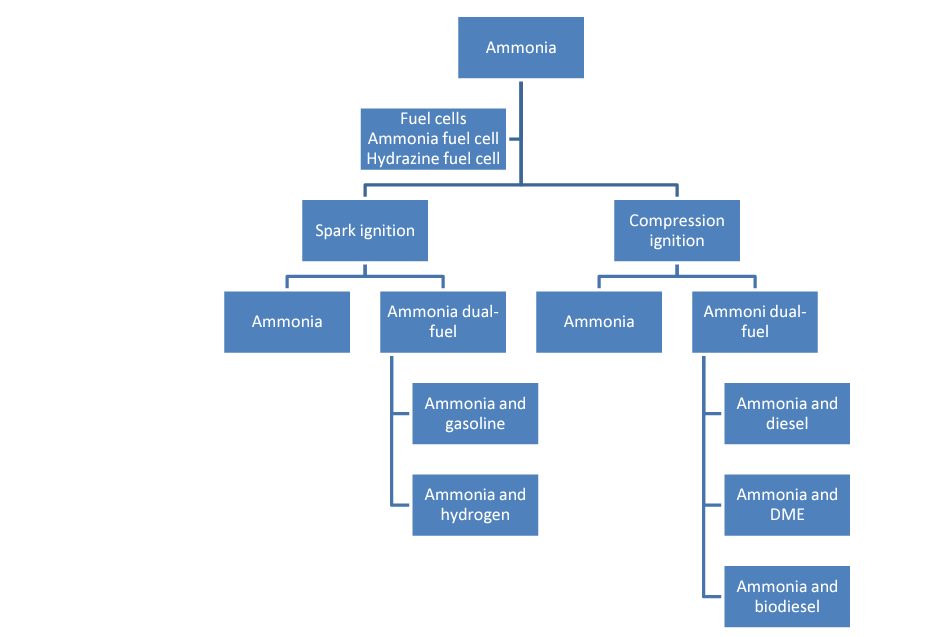
Different fuels can be mixed with ammonia for Otto cycle, or gasoline, and carnot cycle, or diesel engines, in order to facilitate combustion. The following figure shows the categories of possible fuel mixtures for use in different propulsion technologies, such as fuel cells, gasoline engines ("Spark Ignition") and diesel engines ("Compression Ignition").
AMMONIA IN DIESEL ENGINES
MONOFUEL
The exclusive use of ammonia as fuel in an internal combustion engine is not possible due to the high compression ratios necessary for ignition/combustion. Very high compression ratios, up to 35:1, are needed to use ammonia as fuel in internal combustion engines. Liquid ammonia used in internal combustion engines does not combust with compression ratios up to 30:1. A standard diesel engine runs with a compression ratio of 15 to 17:1
DUALFUEL
Ammonia content of up to 95% was feasible with only 5% diesel with a John Deere engine. But the optimal mixture is 40% diesel - 60% ammonia, since a higher amount of diesel would limit the flammability of ammonia.
An engine tested with biodiesel and ammonia as fuel performs in the same way as one with diesel and ammonia with the same performance characteristics. But the performance characteristics differ when dimethyl ether (DME) is used with ammonia as fuel, with an ammonia content of up to 80% being feasible.
AMMONIA IN GASOLINE ENGINES
MONOFUEL
Combustion of ammonia in a spark-ignition engine can be facilitated with a more compact combustion chamber and longer spark plugs to overcome ammonia's reluctance to burn.
DUALFUEL
Gasoline as a combustion promoter requires a compression ratio of 10:1 for optimal operation at 30% gasoline content.
Liquid ammonia would reduce the temperature in the cylinder and thus make it more difficult for subsequent turbulence, which would lead to impaired combustion and misfiring. For this reason, gaseous ammonia is directly injected and would be more easily usable, while gasoline is injected to improve combustion. Idling requires 100% gasoline, but can be reduced to 20% under running conditions.
AMMONIA FUEL CELLS
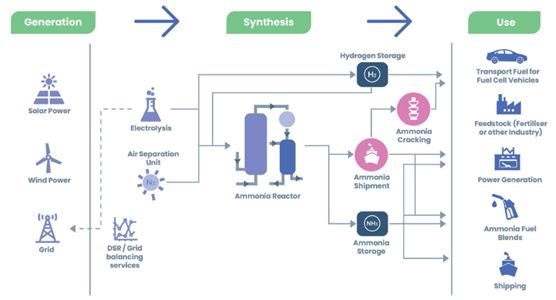
Theoretically, solid oxide fuel cells could be used directly with ammonia, saving the high pressures necessary for the same use with hydrogen, however, due to the high temperatures that these fuel cells reach, more than 500ºC, they do so unfeasible for use on the road, although it could be applied in maritime transport.
On the other hand, there is the possibility that is already being explored by various companies of using ammonia as a vector for hydrogen storage. To do this, the ammonia is separated into hydrogen and nitrogen by means of a cracker, and then the H2 is used in a hydrogen fuel cell to produce electricity that will be used to move the vehicle.

Advantages of using ammonia in vehicles
Basically, the advantages of using ammonia would be three:
- First of all, NO CO2 is emitted. Being an element without carbon, the reaction to produce carbon dioxide cannot be produced chemically.
- Second, ease of use. On the one hand, ammonia is already the most widely used chemical on the planet, it is produced on a large scale and is easily manageable in liquid form at low pressures.
- Third, the price can be competitive with traditional fuels. Already today the production price of ammonia is around 250 dollars per ton. It is expected that the production of a green ammonia, the production of ammonia requires high amounts of energy, could be competitive in price with the rest of the fuel.